The article below reflects the personal opinions of the author(s) and does not reflect the views or opinions of the Perspectives editors or committee, or the National Society of Genetic Counselors (NSGC).
Article authored and provided by GeneDx as part of a paid partnership with NSGC. The content, views and opinions expressed in this article are those of GeneDx, and do not necessarily reflect the opinions and views of the National Society of Genetic Counselors.
Cerebral palsy (CP) is one of the most common motor disabilities in children, with an estimated 10,000 children diagnosed each year.1,2,3 Many children with cerebral palsy also experience overlapping comorbidities, with the most common being epilepsy, intellectual disability, speech disorders and autism.4 Until recently, the primary causes of CP were assumed to be perinatal risk factors, including prematurity or birth complications such as asphyxia or trauma. However, a recent systematic review of individuals with CP estimated that an underlying genetic condition is identified in up to one-third of individuals tested. The diagnostic yield increased to 42% when excluding individuals with a known CP risk factor, such as perinatal complications or brain malformations, but was still 21% in unselected CP cohorts.5
Illustrating its genetic heterogeneity, a growing number of clinically relevant genes have been identified in individuals with CP, with recent estimates highlighting over 300.6 Like many other disease-causing gene discoveries, advances in our understanding of the causes of CP have been largely due to the utilization of exome sequencing (ES).1 Comprehensive genetic testing, such as ES or genome sequencing (GS), can play an important role in the care of individuals with CP. With a genetic diagnosis, clinical interventions can be tailored to the affected individual and medical management changes can be made in areas such as surveillance, genetic counseling, therapy services and more.7
Perspectives From a Genomic Sequencing Lab
In the past 10 years, nearly 10,000 individuals with CP underwent clinical ES or GS at GeneDx. Approximately 95% of individuals had at least one comorbidity in addition to CP, including seizures, intellectual disability (ID), developmental delay (DD) or autism. Because ES is already recommended as a first-tier test for individuals with neurodevelopmental disorders (NDD), diagnostic rates were evaluated with and without these comorbidities and are shown in Figure 1, subgrouped by the age of testing. While diagnostic rates were higher for individuals with CP who had NDD or seizure comorbidities, rates were still between 14% and 18% for those with CP who did not have seizures, ID, DD or autism reported. These diagnostic yields are similar to what is seen in cohorts of individuals with NDDs8. ES/GS analysis has many benefits over gene panel testing, including the identification of promising candidate genes and potential for future reanalysis. Our cohort emphasizes the importance of a comprehensive ES or GS approach to genetic testing for individuals with CP, no matter their age or comorbidities.
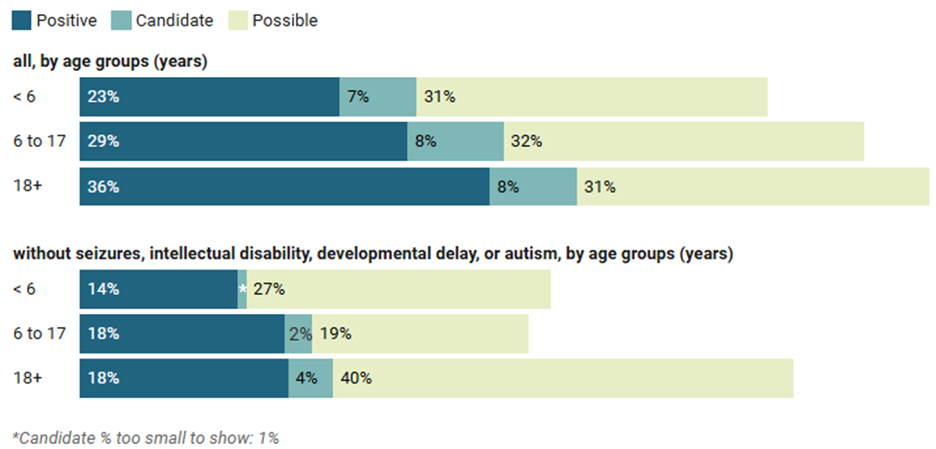
Figure 1: Proportion of individuals with cerebral palsy (CP) receiving positive, candidate, or possible genetic findings, stratified by age at exome or genome sequencing and overlapping comorbidities (internal dataset).
Implications for Clinical Management
Pathogenic variants in the COL4A1 gene have been identified by an expert panel as having high confidence to be related to a gold standard clinical definition of CP.6 COL4A1-related disease causes a spectrum of related disorders with overlapping phenotypes including type I porencephaly, brain small-vessel disease with hemorrhage, brain small-vessel disease with Axenfeld-Rieger anomaly, and hereditary angiopathy with nephropathy, aneurysms, and muscle cramps (HANAC) syndrome.9,10 Individuals with disruption of COL4A1 or COL4A2 are sometimes referred to as having Gould syndrome.
Gould syndrome should be considered in the differential diagnosis for individuals and families with early-onset cerebrovascular disease, certain structural brain anomalies, intracerebral hemorrhage, hematuria, kidney failure, optic abnormalities and cerebral palsy.11 A recent publication outlined diverse diagnostic, screening and management guidelines for affected individuals and included neurologic, cerebrovascular, ophthalmologic, cardiovascular, genitourinary, hematologic and musculoskeletal system recommendations.11 More information on this syndrome can be found through The Gould Syndrome Foundation.
Within our cohort of individuals with CP who had ES or GS, 8,100 individuals were younger than 18 years old at the time of testing. Among these individuals, pathogenic variants in COL4A1 were the most common single-gene findings. While many ES/GS test results in our cohort were considered clinically actionable, COL4A1 disorders are of special note given their complexity and recommended management by a large multidisciplinary team.11
Comprehensive genomic testing should be considered for all individuals with CP, regardless of the age, presumed cause or lack of additional neurodevelopmental or neurologic findings. The potential to identify an underlying genetic condition can reduce self-blame, provide answers for the family and lead to a more targeted care pathway for the affected individual. For many individuals, a diagnosis of cerebral palsy occurs early in childhood. With clinically actionable genetic test results reported in up to 37% of cases5,12, incorporating ES or GS as an early diagnostic test allows for the potential to implement interventions that could have a positive impact on health outcomes at a young age.
References
- Fehlings DL, Zarrei M, Engchuan W, et al. Nat Genet. 2024 Apr;56(4):585-594., Srivastava S, Lewis SA, Cohen JS, et al. JAMA Neurol. 2022 Dec 1;79(12):1287-1295., Lewis SA, Ruttenberg A, Iyiyol T, et al. EBioMedicine. 2024 Aug;106:105229.
- Durkin MS, Benedict RE, Christensen D, et al. Pediatric Perinat Epidemiol. 2016 Sep;30(5):496-510.
- Maenner MJ, Blumberg SJ, Kogan MD, et al. Ann Epidemiol. 2016 Mar;26(3):222-6.
- Van Eyk CL, Fahey MC, Gecz J. Nat Rev Neurol. 2023 Sep;19(9):542-555
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, Finucane BM, Ledbetter DH, Martin CL, Moreno-De-Luca. JAMA Pediatr. 2023 May 1;177(5):472-478.
- Wilson YA, Garrity N, Smithers-Sheedy H, et al. J Child Neurol. 2024 Nov;39(13-14):500-509. doi: 10.1177/08830738241277231. Epub 2024 Sep 9
- Srivastava S, Cole J, Cohen J, et al. Ann Neurol. 2024 96:900-913.
- Srivastava S, Love-Nichols JA, Dies KA, et al; NDD Exome Scoping Review Work Group. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med. 2019;21(11):2413-2421.
- Plaisier E, Ronco P. COL4A1-Related Disorders. 2009 Jun 25 [updated 2016 Jul 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025.
- Kuo DS, Labelle-Dumais C, Gould DB. Hum Mol Genet. 2012 Oct 15;21(R1):R97-110. doi: 10.1093/hmg/dds346. Epub 2012 Aug 21.
- Tambala D, Vassar R, Snow J, et al. Genet Med. 2025 Sep;27(9):101514. doi: 10.1016/j.gim.2025.101514. Epub 2025 Jul 2.
- Lewis SA, Chopra M, Cohen JS, et al.Clinical Actionability of Genetic Findings in Cerebral Palsy: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2025 Feb 1;179(2):137–44. doi: 10.1001/jamapediatrics.2024.5059.
Bobbi McGivern, MS, CGC (she/her) Bobbi McGivern is a clinical program manager in clinical and translational research at GeneDx. She has been an ABGC board-certified genetic counselor since 2005 and has spent nearly 20 years working in clinical practice. Over the past year, she has coauthored more than a dozen peer-reviewed publications. To further expand her expertise in research, she is pursuing a graduate certificate in health economics and outcomes research.