The article below reflects the personal opinions of the author(s) and does not reflect the views or opinions of the Perspectives editors or committee, or the National Society of Genetic Counselors (NSGC).
Article authored and provided by Ambry Genetics as part of a paid partnership with NSGC. The content, views and opinions expressed in this article are those of Ambry Genetics, and do not necessarily reflect the opinions and views of the National Society of Genetic Counselors.
Exome reanalysis is an evolving standard of care in rare disease genetics, driven by our expanding understanding of gene-disease associations. Clinical exome sequencing evaluates approximately 20,000 genes; however, only about 5,000 to 6,000 of these are currently well-characterized. With roughly one new gene linked to disease every two days, exome data can become outdated within just a few years.
Understanding Exome Reanalysis in Practice
One unique benefit of exome and genome testing is the ability to revisit the initial data and reevaluate it for new, clinically relevant findings. Indeed, guidelines from the American College of Medical Genetics and Genomics (ACMG) recommend considering exome reanalysis every one to two years due to the rapid expansion of genomic knowledge and the discovery of new disease-associated genes and variants.¹
Traditionally, reanalysis is initiated when a patient’s phenotype evolves or when sufficient time has passed to justify a fresh review. However, in many clinical settings, the responsibility still falls on the provider — often the genetic counselor — to monitor timelines, track eligibility and submit updated documentation. This process can be time-consuming, administratively complex and easily deprioritized amid a busy clinical workflow.
So, how can laboratories better support genetic counselors and the patients they follow over time?
A Laboratory-Driven Model for Reanalysis
At Ambry Genetics, we’ve shifted the paradigm. Through our Patient for Life™ program, exome reanalysis is no longer entirely provider-dependent. This lab-driven model proactively flags and reinterprets stored exome data whenever new gene-disease associations or variant classifications emerge — often years after the original test was completed.
When clinically significant new findings are identified, Ambry automatically issues an amended report. A local Genomic Science Liaison (GSL) then reaches out to the ordering provider to walk through the update and discuss its implications. This approach minimizes the need for clinicians to track reanalysis timelines or submit routine reanalysis requests — especially when the new findings stem from broader discoveries in the scientific literature.
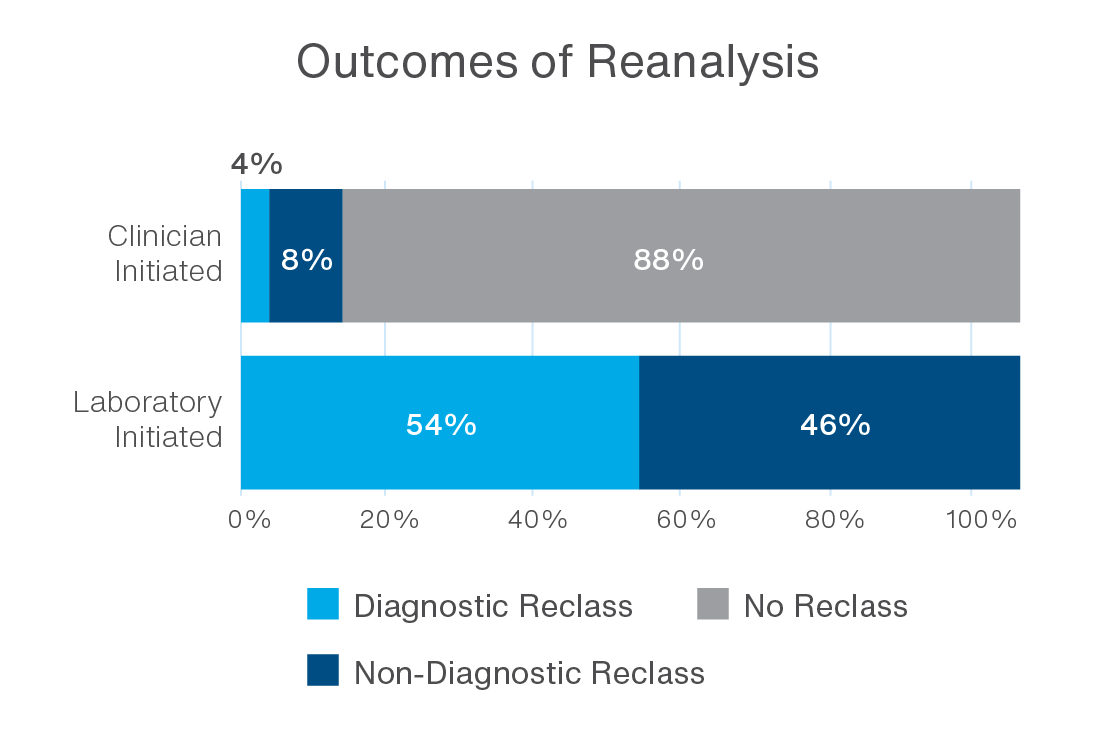
Newly published data from Ambry’s Patient for Life™ program demonstrate the power of a lab-driven approach. Reanalysis initiated by the laboratory led to a 54% diagnostic reclassification rate (p < 0.0001), compared to just 4% for clinician-initiated requests.2 Most provider-initiated reanalyses did not result in any reclassification, emphasizing the value of continuous, proactive review in identifying clinically relevant updates.
Supporting Longitudinal Care Without Added Burden
For genetic counselors, the Patient for Life™ model offers tangible benefits:
- Reduces administrative workload by eliminating routine reanalysis requests.
- Prevents missed diagnostic opportunities in patients who may be lost to follow-up.
- Improves long-term patient care by integrating new scientific findings without requiring a new sample or test.
- Fosters stronger lab-clinic collaboration through direct outreach and case discussions.
Importantly, this proactive reanalysis service is included at no additional cost — a critical factor in equitable care for families with ongoing diagnostic needs.
When Provider-Initiated Reanalysis Plays a Key Role
Ambry’s Patient for Life™ program helps alleviate the routine burden of reanalysis by proactively identifying and reporting new clinically relevant findings. Still, there are important scenarios where provider-initiated reanalysis adds critical value.
For example, if a patient’s phenotype evolves, new symptoms emerge or additional clinical evaluations (e.g., imaging or specialist assessments) provide further insight, this updated information can significantly impact how existing exome data is interpreted. In such cases, provider input plays a key role in guiding more personalized analysis.
Submitting a reanalysis request is simple. Providers can use the online form available on Ambry’s website, along with any relevant clinical updates or test results. Once received, our team will complete a reanalysis and issue either:
- A reanalysis notice if no clinically significant changes are found, or
- An amended report if new findings or variant reclassifications are identified.
Turnaround time is currently six to eight weeks, and all reanalysis services are provided at no additional cost.
Reanalysis as a Core Element of Exome Testing
In an era where genetic testing is expected to evolve alongside scientific discovery, GCs need reliable lab partners who view reanalysis as a standard service, not an afterthought. The Patient for Life™ program exemplifies how laboratories can meaningfully support long-term patient care while also reducing the pressure on providers to manually track updates or chase evolving data.
As genomic medicine becomes increasingly personalized and dynamic, Ambry remains committed to building infrastructure and services that empower clinicians — especially genetic counselors — to focus on what matters most: their patients.
Learn more about our Patient for Life program.
References
- Deignan JL, Chung WK, Kearney HM, et al. Points to consider in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2019;21(6):1267-1270. doi:10.1038/s41436-019-0478-1
- Towne MC, Huang J, Saliganan S, et al. Impact of laboratory-driven proactive reanalysis: reclassification to positive in 5% of initially negative or uncertain exome sequencing cases. Genet Med. 2025;101464. doi:10.1016/j.gim.2025.101464
Melissa Holman, MS, CGC Melissa Holman is a certified genetic counselor and cardiology & rare disease genomic Science Liaison at Ambry Genetics. She completed her B.S. in genetics with a minor in psychology at Clemson University and received her Master of Science in Genetic Counseling from Virginia Commonwealth University. After graduate school, Melissa worked for several years as a genetic counselor in South Bend, Indiana. In 2019, she moved to Winston Salem, North Carolina, and transitioned to her current role at Ambry Genetics. As a genomic science liaison, she serves as a clinical liaison for the field team to educate health care providers and other key opinion leaders on genetic testing and genomic medicine.